
Oceans of diamond possible on Uranus and Neptune
DR EMILY BALDWIN
ASTRONOMY NOW
Posted: 21 January 2010


High pressure experiments that mimic conditions on the icy gas giants show that chunks of diamond can float on a sea of liquid carbon.
The research provides the first detailed measurements of the melting point of diamond, the hardest natural material known, and the finding that could also help explain the strange orientation of Uranus and Neptune's magnetic fields.

 Uranus (left) and Neptune (right), as seen by the Voyager 2 spacecraft. Image: NASA/JPL. Uranus (left) and Neptune (right), as seen by the Voyager 2 spacecraft. Image: NASA/JPL.
The existence of pure carbon in the interiors of these giant planets has gained both experimental and theoretical support in recent years, and understanding the high pressure and temperature behaviour of carbon is essential to predicting their evolution and structure. Current theories speculate that Neptune and Uranus have solid cores surrounded by an icy mantle of water, ammonia and methane ices.
In the new experiment, led by Jon Eggert of the Lawrence Livermore National Laboratory, scientists blasted diamonds just two millimetres in diameter and 0.5 millimetres thick with a powerful laser – the Omega laser at the University of Rochester, New York – to reach temperatures and pressures of 110,000 Kelvin and 4,000 giga Pascals respectively. The pressure, which is equivalent to 40 mega bars, is 40 million times greater than what a person feels when standing at sea level on the Earth.
As the scientists watched the pressure decay they saw that the temperature increased. "This implied a large energy sink which we interpret to be melting of diamond," Eggert tells Astronomy Now. "What is really neat is that we could measure the temperature and pressure of the diamond-melt mixture over a large pressure range from about 6 to 11 million atmospheres." Over this pressure range the diamond showed behaviour much like water in the sense that the solid component was less dense than the liquid – the scientists saw tiny chunks of diamond floating in a sea of liquid carbon, just as ice floats on water.
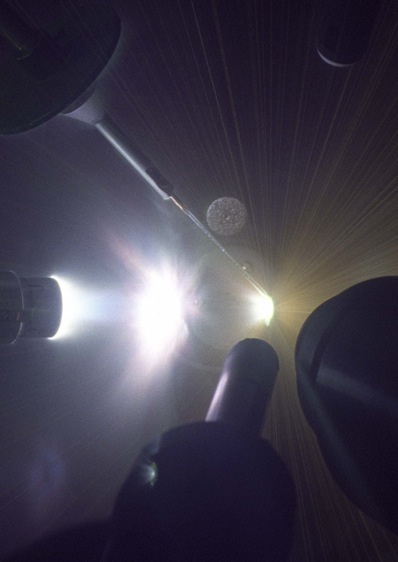 Time-integrated photograph of an OMEGA laser shot to measure high-pressure diamond melt. The diamond target is at centre right, bright white light is ablated plasma, and radial yellow lines are tracks of hot target fragments very late in time. Image: Eugene Kowaluk, LLE. Time-integrated photograph of an OMEGA laser shot to measure high-pressure diamond melt. The diamond target is at centre right, bright white light is ablated plasma, and radial yellow lines are tracks of hot target fragments very late in time. Image: Eugene Kowaluk, LLE.
While providing new understanding of the behaviour of diamond at high pressures, the results can also be applied to the conditions that prevail inside ice giants Neptune and Uranus. Could we expect great seas of diamonds in the outer reaches of our Solar System? "This is a very speculative scenario," says Eggert. "I think it could be more like a liquid carbon core surrounded by floating diamond or possibly 'diamond-bergs'. Whether the diamond would break up into chunks or bergs is pure speculation at this point. It should also be remembered that there would be a whole planet of hydrogen and helium on top of this carbon ocean, but if there was diamond it would be the first solid found in these planets."
A swirling internal ocean of diamond could explain the long-standing mystery as to why the ice giants' magnetic poles are offset from the geographic poles by up to 60 degrees. Planetary magnetic fields are generated by complex fluid motions in electrically conducting regions of the planet and the diamond could deflect or tilt the field to match the observed orientation of these planets' magnetic fields.
However, there are still many pieces to fill in before fully understanding the internal structure of these outer Solar System planets and what is driving their magnetic fields. "It is very likely that at the temperatures and pressures near the centre of Neptune/Uranus the molecules are unstable and break up, so that the water, ammonia, methane mixtures in the upper planet dissociate into hydrogen, carbon, nitrogen, oxygen and helium at depth," says Eggert. "These elements may then mix or separate. This has had little or no experimental testing because it is only very recently that we could obtain these pressures and temperatures in the laboratory to do these experiments, but these types of mixtures are definitely on our list of experiments to do in the future!"
|



